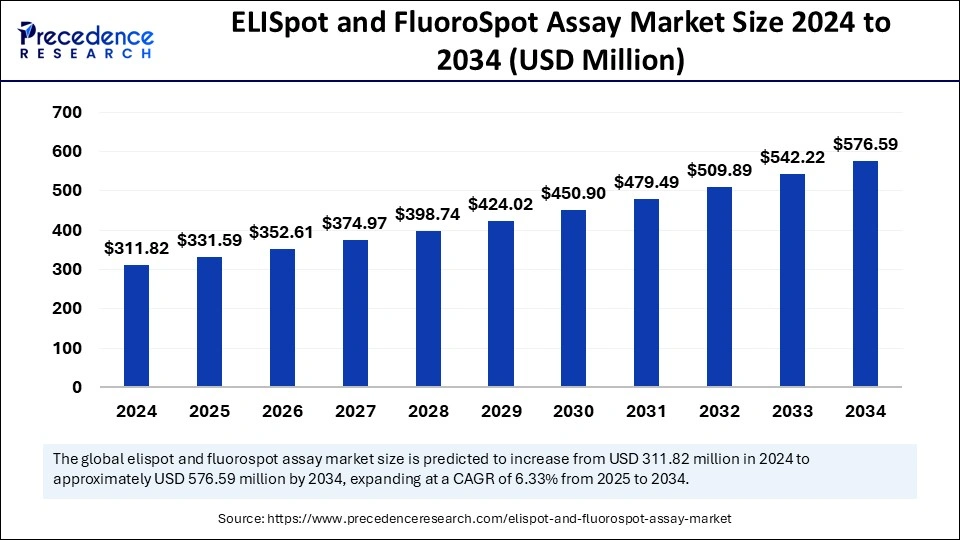
The global ELISpot and FluoroSpot assay market size surpassed USD 311.82 million in 2024 and is projected to surge around USD 576.59 million by 2034 with a CAGR of 6.33%.
ELISpot and FluoroSpot Assay Market Key Points
-
In 2024, North America held the top position globally with a market share of 35%.
-
Asia Pacific is poised to achieve the highest CAGR of 7.24% during the forecast period.
-
Europe is likely to record substantial gains in market share in the coming years.
-
The assay kits segment was the market leader by product in 2024, securing a 50% share.
-
The analyzers product category is expected to exhibit rapid growth between 2025 and 2034.
-
Diagnostic applications led the market in 2024, comprising 67% of the total share.
-
Research applications are projected to expand at the fastest CAGR over the next several years.
-
Hospitals and clinical labs remained the top end-user segment in 2024 with a 47% contribution.
-
Biopharmaceutical companies are likely to see the most rapid growth among end-use sectors through the forecast period.
The Transformative Role of AI in the ELISpot and FluoroSpot Assay Market
Artificial Intelligence (AI) is playing a transformative role in the ELISpot and FluoroSpot Assay Market by enhancing accuracy, speed, and scalability in immune monitoring and cell-based assays. AI-powered image analysis tools enable precise detection and quantification of cytokine-secreting cells by distinguishing true signals from background noise. This significantly reduces manual errors and increases the reproducibility of results, especially in high-throughput environments such as clinical diagnostics, vaccine development, and immunotherapy research.
Beyond image analysis, AI also streamlines workflow automation and data interpretation. By integrating assay results with multi-omics data and predictive models, AI facilitates deeper insights into immune responses and helps identify disease-specific biomarkers. Additionally, cloud-based AI platforms support remote analysis and centralized data management, making collaboration across research sites more efficient. As a result, AI integration is emerging as a key driver of innovation and competitiveness in the ELISpot and FluoroSpot market.
What is ELISpot?
ELISpot (Enzyme-Linked ImmunoSpot) is a highly sensitive, cell-based assay used to detect and quantify individual immune cells secreting specific cytokines or other proteins. It is primarily used to monitor immune responses, especially T-cell activity, by measuring how many cells in a sample produce a particular cytokine such as IFN-γ or IL-2.
Key Features:
-
Detects cytokine secretion at the single-cell level
-
Provides quantitative and functional data on immune cells
-
Widely used in vaccine development, infectious disease research, autoimmune studies, and cancer immunology
What is FluoroSpot?
FluoroSpot is an advanced version of ELISpot that uses fluorescent detection instead of enzymatic color change. This allows simultaneous detection of multiple cytokines from a single cell, enabling multiplexing in one assay.
Key Features:
-
Detects polyfunctional cells secreting multiple cytokines
-
Uses fluorescent antibodies to identify different cytokines in one well
-
Ideal for complex immune profiling, especially in studies involving T-cell polyfunctionality
Applications of Both Assays:
-
Vaccine efficacy studies (such as COVID-19, HIV, and tuberculosis)
-
Immunotherapy research in cancer and autoimmune diseases
-
Drug development and clinical trials
-
Transplantation research to monitor immune rejection or tolerance
Market Overview
The ELISpot and FluoroSpot Assay market is gaining momentum as a key tool in immunological assessments. With the capability to measure cell-mediated immune responses at a single-cell level, these assays have become crucial in understanding disease mechanisms and evaluating immune responses in therapeutic interventions.
ELISpot and FluoroSpot Assay Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 576.59 Million |
| Market Size in 2025 | USD 331.59 Million |
| Market Size in 2024 | USD 311.82 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.33% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product , Application, End Use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
ELISpot and FluoroSpot Assay Market Dynamics
Market Drivers
The increasing incidence of chronic and infectious diseases is a major driver of market demand. Growth in biopharmaceutical R&D, particularly in cancer and infectious disease vaccines, along with advancements in assay technologies, are further propelling the market forward.
Opportunities
There is significant growth potential in emerging markets where investments in healthcare infrastructure are increasing. Innovations in assay platforms, particularly those that support multiplexing and AI-assisted analysis, open new pathways for application in translational research and clinical diagnostics.
Challenges
The complexity of data interpretation, especially in multi-cytokine FluoroSpot assays, and the high cost of reagents and analyzers are notable challenges. Additionally, adoption in low-resource settings may be limited due to infrastructure constraints and lack of trained staff.
Regional Insights
North America leads the global market owing to a high concentration of pharmaceutical companies and advanced research facilities. Asia Pacific is projected to be the fastest-growing region due to expanding clinical trial activities and supportive regulatory environments. Europe also holds a significant share, with continued investment in life sciences research.
ELISpot and FluroSpot Assay Market Comapnies

- Abcam Limited
- Bio-Techne Corporation0
- BD
- Cellular Technology Limited
- Mabtech
- Autoimmun Diagnostika GmbH
- Lophius Biosciences GmbH
- Bio-Connect B.V.
- Oxford Immunotec
- U-CyTech
Leader’s Announcements
- In March 2025, Beckman Coulter Diagnostics, announced the U.S. FDA 510 (k) clearance for their novel DxC 500i Clinical Analyzer which is an integrated clinical chemistry and immunoassay analyzer. Kathleen Orland, Chief Portfolio Officer for Beckman Coulter Diagnostics, said that, “Innovations like the DxC 500i Clinical Analyzer enable Beckman Coulter to address the needs of networked laboratories with specific solutions for satellite or independent laboratories, as well as core laboratories. Beyond ensuring appropriate throughput levels for a networked lab, Beckman Coulter’s common reagents and consumables across its scalable clinical chemistry and immunoassay portfolio enables common reference ranges, offering IDNs strategic benefits in patient care and inventory management.”
- In January 2025, CellFE, a disruptive microfluidics company leading in non-viral gene editing technology announced the launch of its CellFE T-Rest (Resting T Cell Kit) which is a cell manufacturing transfection media product designed specifically for resting (quiescent) T cell workflows. Alla Zamarayeva, CEO of CellFE, said that, “We’re thrilled to launch T-Rest – the first-in-class resting T cell commercial product – to offer a new paradigm for cell therapy manufacturing. By enabling efficient editing of resting T cells, we address the durability and safety concerns that currently challenge cell therapy manufacturers.”
Recent Developments
- In October 2024, BioVaxys Technology Corp., a clinical stage biopharmaceutical company, declared that DPX, an innovative immune educating delivery platform developed by the company assists in recruiting and activating unique subsets of antigen presenting cells (APCs) for accelerating the immunogenicity of antigens further showing enhanced immune activation in comparison to aqueous and emulsion-based antigen delivery systems.
- In May 2024, Revvity Inc., a life sciences and diagnostics company, launched the Auto-Pure 2400 liquid handler from Allsheng which can be utilized with the T-SPOT.TB test for detecting latent tuberculosis infection (LTB1). The test is an interferon-gamma release assay (IGRA) predicated on the enzyme-linked immunospot (ELISPOT) technology and the Auto-Pure 2400 automated liquid handling platform is laced with integrated magnetic cell isolation technology.
Segments Covered in the Report
By Product
- Assay Kits
- Analyzers
- Ancillary Products
By Application
- Research Applications
- Diagnostics Applications
By End-use
- Hospital and Clinical Labs
- Academic and Research Institutes
- Biopharmaceutical Companies
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Also Read: Micro Mobile Data Center Market
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/